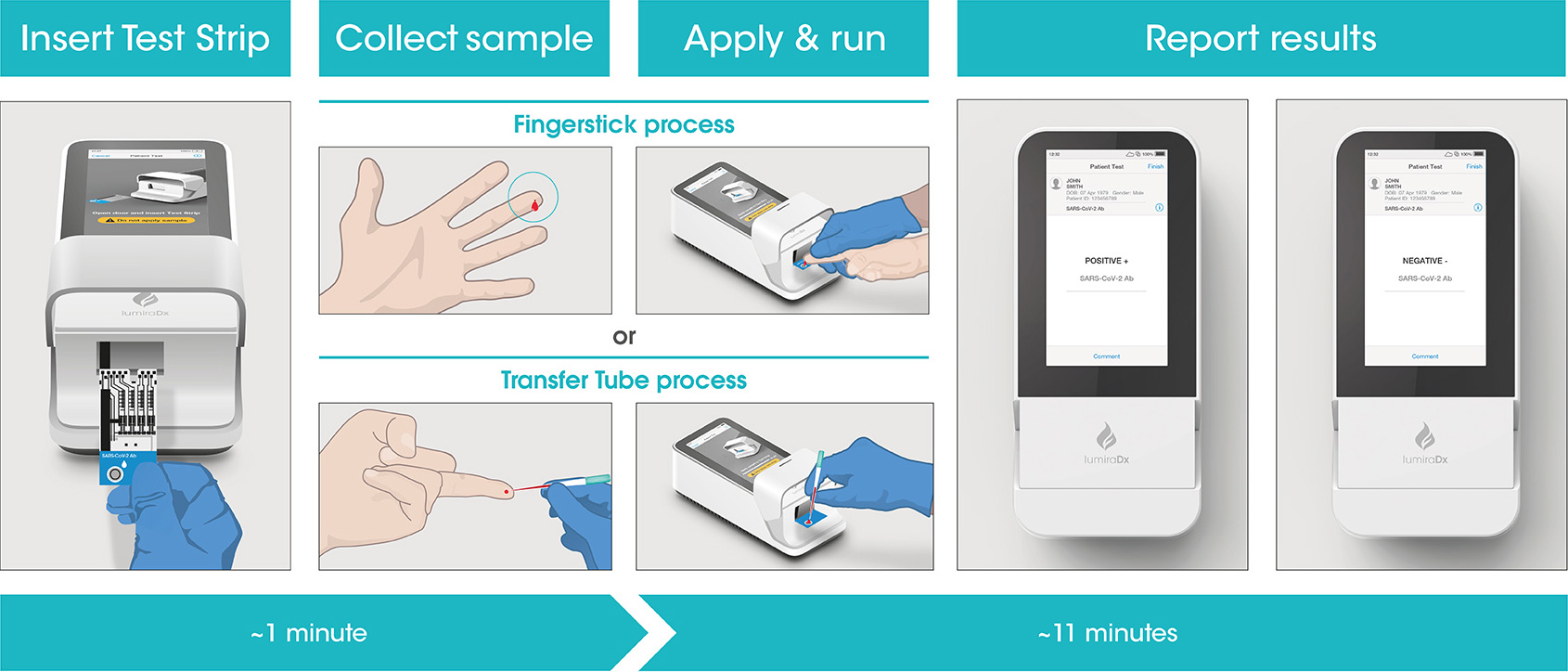
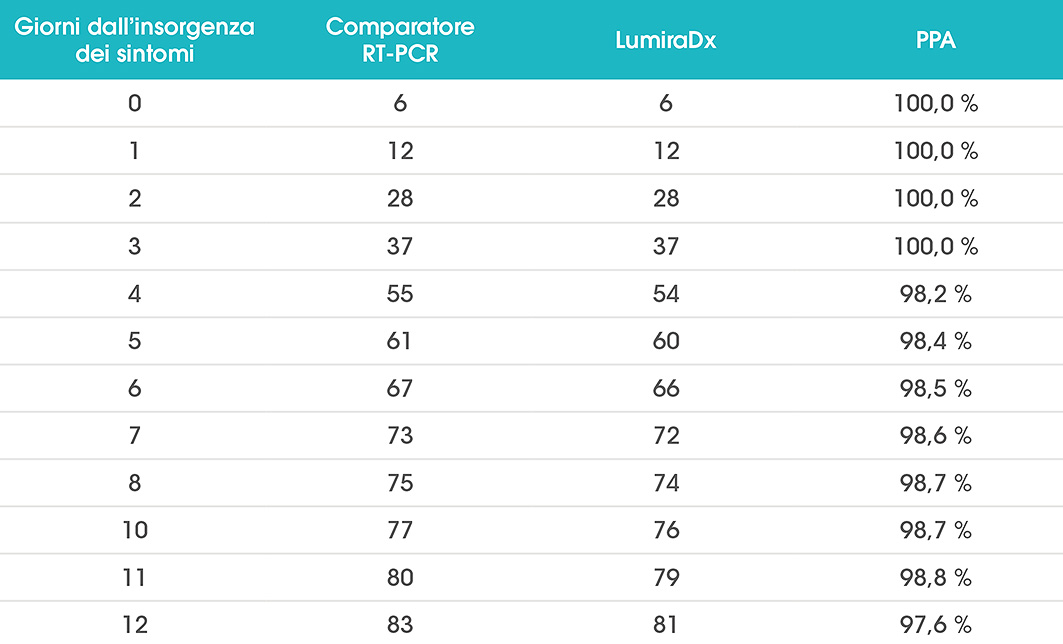
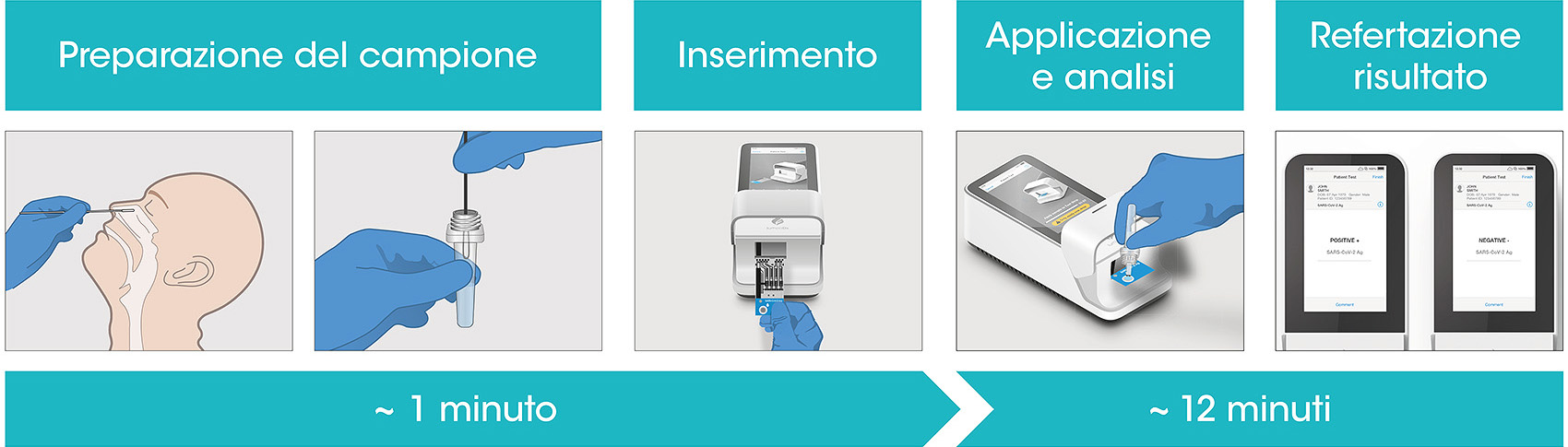
LumiraDx Antigen Test Supports Established Testing Concepts with Rapid and Accurate Results in Germany

Test Covid 19 Ultima Generazione, Immunofluorescenza con lettura microfluidica - Farmacia Pescetto di Pegli, Genova

LumiraDx riceve l'autorizzazione per il test antigenico SARS-CoV-2 in Giappone e Brasile; l'Italia raccomanda l'espansione del test antigenico basato su microfluidica

A rapid, high-sensitivity SARS-CoV-2 nucleocapsid immunoassay to aid diagnosis of acute COVID-19 at the point of care | medRxiv